EASE
Efficacy and safety of 8- versus 12-week sofosbuvir-ravidasvir treatment for non-cirrhotic chronic hepatitis C patients: An open-label, randomized, multicenter study in Malaysia
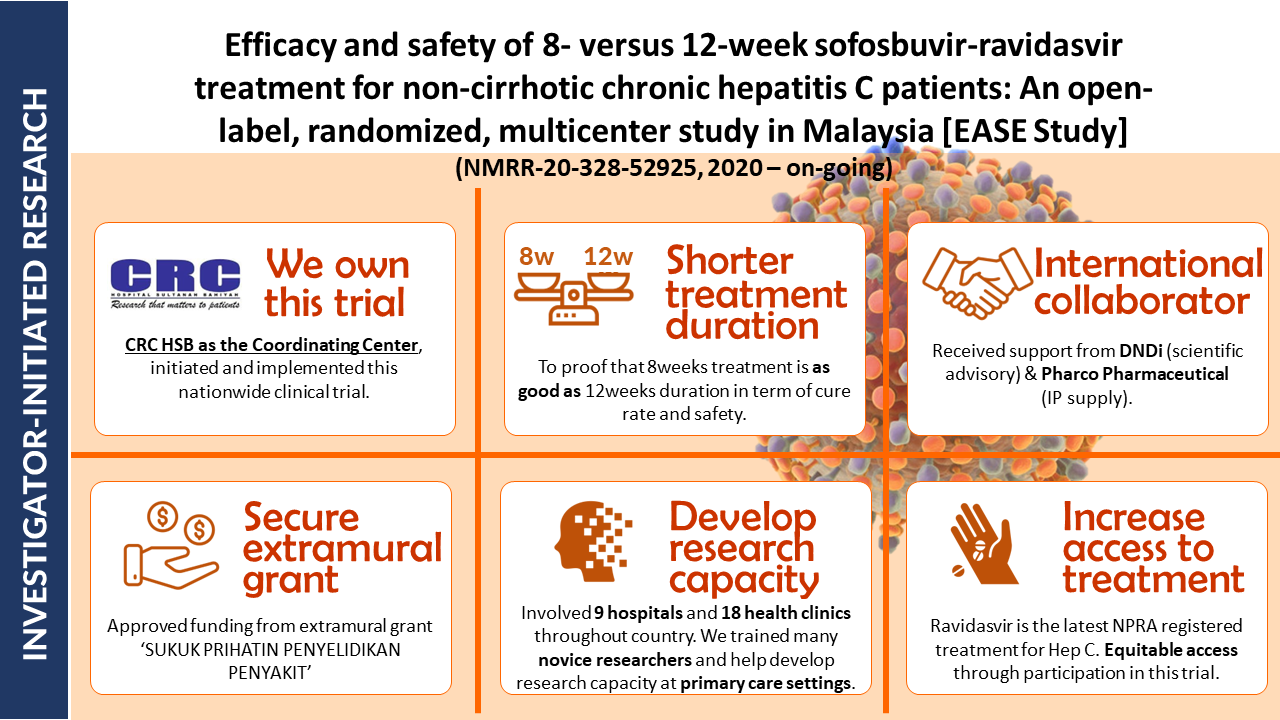
Study overview: A Pioneering Malaysian Study Investigates the Efficacy of an 8-Week SOF-RVD Combination Treatment for Non-Cirrhotic Chronic Hepatitis C. This multicenter, open-label, randomized controlled trial represents a significant advancement in Hepatitis C treatment strategies. The study, wholly designed and implemented by the Malaysian Ministry of Health, compares the efficacy and safety of an 8-week versus a 12-week regimen of SOF-RVD combination therapy for patients with non-cirrhotic chronic Hepatitis C. All enrolled subjects will receive the designated treatment and be monitored for a 24-week follow-up period following treatment completion.
This groundbreaking research holds the potential to revolutionize Hepatitis C treatment by:
- Enhancing Patient Adherence: A shorter treatment duration is hypothesized to significantly improve patient compliance, particularly among vulnerable populations such as people who inject drugs (PWID).
- Expanding Treatment Accessibility: The study's inclusion of primary care health clinics provides a more accurate representation of the Hepatitis C patient population in Malaysia. This decentralized approach has the potential to broaden access to treatment across the nation.
- Promoting Non-Invasive Assessment: The study's utilization of non-invasive scoring systems (APRI score & FIB-4 index) for liver cirrhosis evaluation facilitates treatment decentralization to primary care settings.
- Empowering Primary Care Physicians: By equipping family physicians with highly effective Direct Acting Antivirals (DAAs) and these accessible assessment tools, the study empowers primary care to deliver comprehensive Hepatitis C care.
- Demonstrating LMIC Leadership: This Malaysian-led initiative underscores the potential for effective drug development and implementation within Low- and Middle-Income Countries (LMICs), independent of major pharmaceutical involvement.
The findings from this pivotal study hold significant implications for the global fight against Hepatitis C. The potential for a shorter, more accessible treatment regimen paves the way for improved patient outcomes and broader access to care, particularly in resource-limited settings.
Number of subjects screened: 473
Number of subjects recruited: 322
Investigator site:
Study sites: 9 public hospitals and 18 health clinics
- Hospital Serdang, Selangor
- Hospital Selayang, Selangor
- Hospital Sultanah Nur Zahirah, Terengganu
- Hospital Sultanah Bahiyah, Kedah
- Hospital Tengku Ampuan Afzan, Pahang
- Hospital Raja Perempuan Zainab, Kelantan
- Hospital Sultanah Aminah, Johor
- Hospital Pulau Pinang
- Hospital Kuala Lumpur
- Klinik Kesihatan Petra Jaya, Sarawak
- Klinik Kesihatan Miri, Sarawak
- Klinik Kesihatan Kuah, Kedah
- Klinik Kesihatan Bandar Sungai Petani
- Klinik Kesihatan Bandar Pusat Jengka, Pahang
- Klinik Kesihatan Tampin, Negeri Sembilan
- Klinik Kesihatan Kuala Besut, Terengganu
- Klinik Kesihatan Batu Pahat, Johor
- Klinik Kesihatan Serendah, Selangor
- Klinik Kesihatan Ampang, Selangor
- Klinik Kesihatan Taiping, Perak
- Klinik Kesihatan Parit, Perak
- Klinik Kesihatan Ayer Tawar, Perak
- Klinik Kesihatan Masjid Tanah, Melaka
- Klinik Kesihatan Bachok, Kelantan
- Klinik Kesihatan Senawang
- Klinik Kesihatan Bayan Lepas, Penang
- Klinik Kesihatan Butterworth, Penang
Sponsor: Ministry of Health Malaysia (MOH) grant
Collaborators: DNDI- Drugs for Neglected Disease Initiative, Geneva & Pharco Pharmaceuticals, Egypt