Clinical Research Centre
Hospital Ampang
Selangor
WhoWe Are
Clinical Research Centre Hospital Ampang (CRC HAMP) is currently headed by Dr. Jerome Tan Tsen Chuen, Consultant Haematologist, since 2019. CRC HAMP continues to grow. CRC consists of 2 staffs of Hospital Ampang, 4 ICR staffs and 7 Clinical Research Malaysia (CRM) staffs. CRC HAMP is located at Level 3 Specialist Office. CRC HAMP is one of the 37 CRC networks under the Institute of Clinical Research (ICR), Malaysia. CRC HAMP was initiated in Year 2007, has grown to become an efficient and accountable centre to conduct quality and ethical research in order to enhance patient outcome. It was established to provide assistance in clinical studies, especially in the promotion of research among Hospital Ampang staff.
OurTeam
InvestigatorsHighlight

Head of CRC, Consultant Clinical Haematologist, Hospital Ampang, Selangor
Email: romeje@gmail.com
MBBS (UM), MMed (NUS), MRCP (UK), Fellowship in Clinical Haematology
Areas of interest: Malignant haematology eg. leukaemias, lymphomas, multiple myeloma and general haematology
Awards and achievements:
- Top recruiter in the EGM for a Follicular Lymphoma trial
Dr. Jerome Tan is a full-time consultant clinical haematologist currently based in the Department of Haematology of Hospital Ampang Selangor, the national referral haematology hospital for the Ministry of Health Malaysia. He also heads the CRC Unit of Hospital Ampang Selangor. He is a certified clinical trainer and supervisor for the national clinical haematology subspecialty fellowship programme for the Ministry of Health, Malaysia. He is also an honorary member of Asia Pacific Leukaemia Consortium and has previously served as a council member of the Malaysian Society of Haematology. Dr. Jerome is a key opinion leader and frequently is invited to speak at various haematology educational and CME programmes nationwide. He is an active clinician and has served in several major hospitals in Sabah, Perak and Selangor. He is also active in various haematology clinical trials as a principal and sub-investigator.
Research Highlights
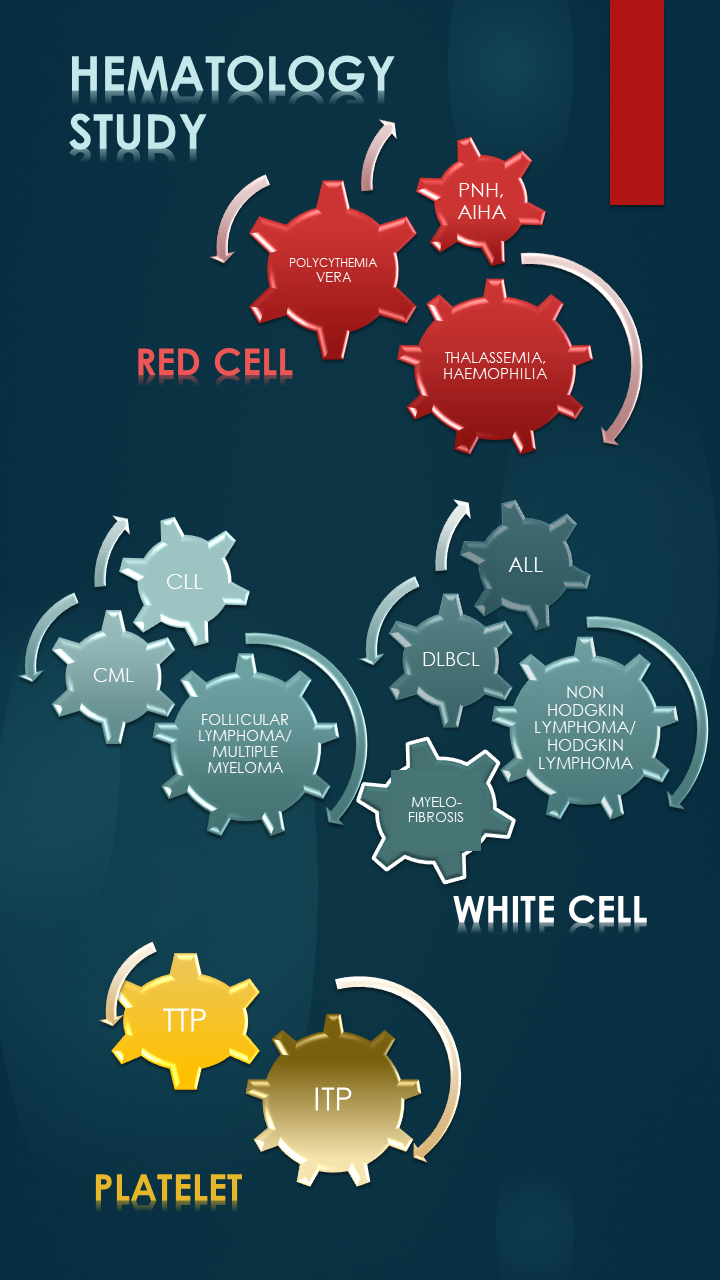
Various industry sponsored Phase 3 clinical trials in Lymphomas, Chronic Myeloid Leukaemia and Acute Myeloid Leukaemia
Recent Publications
Project Highlights
I-TECH trial was conducted in 2021. This study began on May 31, 2021 to evaluate the efficacy of Ivermectin in treating COVID-19 among high-risk patients with mild to moderate symptoms. The study, which included 500 patients was conducted across 21 sites in Malaysia. Participants were divided into two groups: one received a five-day course of Ivermectin along with standard care, while the other group received only the standard care. The primary goal of the study was to assess whether Ivermectin could prevent the progression of COVID-19 to severe disease. The findings revealed that there were no significant differences between the two groups in terms of ICU admissions, need for mechanical ventilation, symptom recovery, or other clinical outcomes. The study also noted that adverse events were more common in the Ivermectin group, with diarrhea being the most frequent side effect.
This study emphasized that despite some early studies suggesting potential benefits, the more robust and larger I-TECH study does not support the use of Ivermectin as a treatment for COVID-19. This study also highlighted that promoting Ivermectin as a "miracle drug" without sufficient evidence is irresponsible, as it could lead to false assurances and delay proper medical treatment.

| NMRR-18-1266-41897 |
A multicentre international randomized parallel group double –blinded placebo-controlled . Clinical trial of EMPAgliflozin once daily to assess cardio-renal outcomes in patients with CKD. EMPA-KIDNEY Trial |
| NMRR-18-1349-39723 | Aldosterone bloCkade for Health Improvement EValuation in End-stage renal disease ACHIEVE |
| NMRR-19-3508-51990 | A multicentre , observational study of patients – Microangiopathic thrombocytopenia by the Asian-Pasific microangiopathic thrombocytopenia (APMAT) NetworkAPMAT 2.0 |
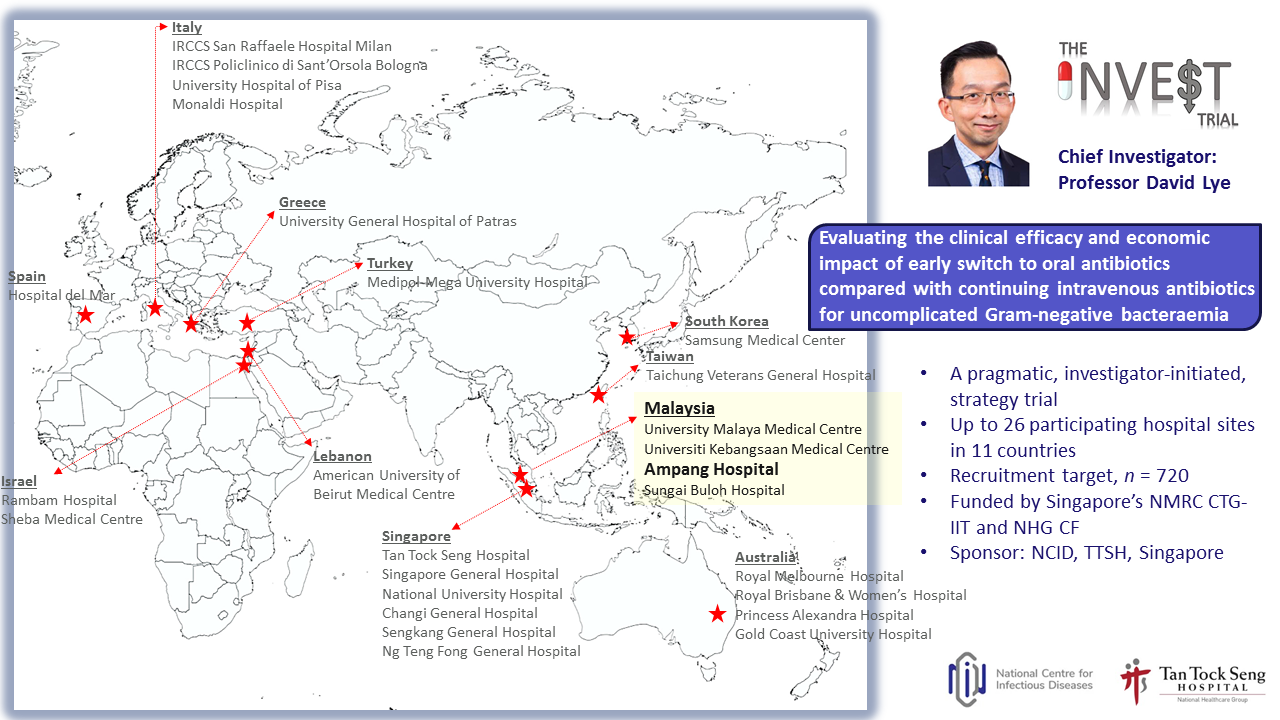
| NMRR-22-02075-TMQ | Early Oral Step-Down Antibiotic Therapy Versus Continuing Intravenous Therapy For Uncomplicated Gram-Negative Bacteraemia (the INVEST trial) INVEST |
| NMRR-23-02467-3JV | Early intravenous to oral antibiotic switch in uncomplicated Staphylococcus aureus bacteraemia (EVOS) |
| NMRR-20-1856-56112 | A Phase III, Randomized, Open-Label, Active-Controlled, Multicenter Study Evaluating The Efficacy And Safety Of Crovalimab Versus Eculizumab In Adult And Adolescent Patients With Paroxysmal Nocturnal Hemoglobinuria (PNH) Not Previously Treated With Complement Inhibitors (BO42162) |
| NMRR-22-00405-WOB | A Randomized, Open-Label, Ravulizumab-Controlled Study to Evaluate the Efficacy and Safety of Pozelimab and Cemdisiran Combination Therapy in Patients with Paroxysmal Nocturnal Hemoglobinuria who are Complement Inhibitor Treatment-Naive or Have Not Recently Received Complement Inhibitor Therapy ( R3918-PNH-2021) |
| NMRR-22-02493-GDM | An Open-Label Extension Study to Evaluate the Long-Term Safety, Tolerability, and Efficacy of Pozelimab and Cemdisiran Combination Therapy in Patients with Paroxysmal Nocturnal Hemoglobinuria R3918-PNH-2050) |
| NMRR-20-3080-56265 | An Open-Label, Single-Arm, Phase 2 Study to Evaluate the Safety, Pharmacokinetics, and Biologic Activity of Pegcetacoplan in Pediatric Patients with Paroxysmal Nocturnal Hemoglobinuria (APL2-PNH-209) |
| NMRR-21-1457-60074 | A phase III, multi-center, open-label, randomized study of oral asciminib versus Investigator selected TKI in patients with newly diagnosed Philadelphia Chromosome Positive Chronic Myelogenous Leukemia in Chronic Phase (CABL001J12301) |
| NMRR-21-1540-59591 | A phase 3b, multi-center, open-label treatment optimization study of oral asciminib in patients with Chronic Myelogenous Leukemia in chronic phase (CML-CP) previously treated with 2 or more tyrosine kinase inhibitors (CABL001A2302) |
| NMRR-18-2297-43730 | An Open Label, Non-Randomized, Multi-Center Extension Study to Evaluate the Long Term Safety and Efficacy of APL-2 in the treatment of Paroxysmal Nocturnal Hemoglobinuria (PNH).(APL2-307) |
| NMRR-17-895-34856 | Phase 3 open-label, multicenter, randomized study of ASP2215 versus salvage chemotherapy in patients with relapsed or refractory Acute Myeloid Leukemia (AML) with FLT3 mutation (2215-CL-0303) |
| NMRR-21-01964-JHU | A Phase 3, Double-blind, Randomized, Placebo-Controlled, Multicenter Study Evaluating the Efficacy and Safety of Mitapivat in Subjects With Non–Transfusion-Dependent Alpha- or Beta-Thalassemia (ENERGIZE) (AG348-C-017) |
| NMRR-22-01999-0L8 | A multinational, open-label, randomised, controlled study to investigate efficacy and safety of NNC0365-3769 (Mim8) in adults and adolescents with haemophilia A with or without inhibitors (frontier 2) |
| NMRR-21-01965-4JL | A Phase 3, Double-blind, Randomized, Placebo-Controlled, Multicenter Study Evaluating the Efficacy and Safety of Mitapivat in Subjects With Transfusion-Dependent Alpha- or Beta-Thalassemia (ENERGIZE-T) (AG348-C-018) |
| NMRR-19-285-46613 | An Open-label, Long-term Safety and Efficacy Study of Fitusiran in Patients with Hemophilia A or B, with or without Inhibitory Antibodies to Factor VIII or IX (LTE15174 |
| NMRR-22-02426-BFW | An Open-label Study to Evaluate the Long-term Safety of BCX9930 Monotherapy in Subjects with Paroxysmal Nocturnal Hemoglobinuria (BCX9930-205) |
| NMRR-21-1540-59591 | A phase 3b, multi-center, open-label treatment optimization study of oral asciminib in patients with Chronic Myelogenous Leukemia in chronic phase (CML-CP) previously treated with 2 or more tyrosine kinase inhibitors (CABL001A2302) |
| NMRR-21-1457-60074 | A phase III, multi-center, open-label, randomized study of oral asciminib versus Investigator selected TKI in patients with newly diagnosed Philadelphia Chromosome Positive Chronic Myelogenous Leukemia in Chronic Phase (CABL001J12301) |
| NMRR-20-2678-56821 | A phase 3, multicenter, randomized, double-blind, placebo-controlled trial comparing the efficacy and safety of tafasitamab plus lenalidomide in addition to R-CHOP versus R-CHOP in previously untreated, high-intermediate and high-risk patients with newly-diagnosed diffuse large B-cell lymphoma (DLBCL) (MOR208C310) |
| NMRR-17-1114-36047 | Cytopeutics® Umbilical Cord Mesenchymal Stem Cells (Cyto-MSC) for Patients with Grade II - IV Acute Graft-Versus-Host Disease: A Phase I/II Clinical Study (POD0030/CP/R) |
| NMRR-15-1121-26592 | Phase 3, prospective, multi-center, open label study to investigate safety, immunogenicity and hemostatic efficacy of PEGylated factor VIII (BAX 855) in previously untreated patients (PUPs) and minimally treated patients (MTPs) < 6 years with severe hemophilia A (FVIII < 1%) (BAX261203) |
| NMRR-19-285-46613 | An Open-label, Long-term Safety and Efficacy Study of Fitusiran in Patients with Hemophilia A or B, with or without Inhibitory Antibodies to Factor VIII or IX (LTE15174) |
| NMRR-19-1963-48953 | Efficacy and Safety of Concizumab prophylaxis in patients with haemophilia A or B with inhibitors (explorer7) |
| NMRR-19-1915-48954 | Efficacy and Safety of Concizumab prophylaxis in patients with haemophilia A or B without inhibitors (explorer8) |
| NMRR-22-01999-0L8 | A multinational, open-label, randomised, controlled study to investigate efficacy and safety of NNC0365-3769 (Mim8) in adults and adolescents with haemophilia A with or without inhibitors (frontier 2) |
| NMRR-23-02387-VRH | Open-label, long-term safety and efficacy study of Mim8 in participants with haemophilia A with or without inhibitors (frontier 4) |
List of Publications associated with CRC HAMP
Services at CRC Hospital Ampang
CRC Hospital Ampang provides training through workshops and consultations:
- Promote research activities :
- Conduct courses such as Introduction to Clinical Research, Basic Biostatistics Workshop and Good Clinical Practice (GCP).
- Conduct regular in - house Continuous Professional Development (CPD).
- Run research clinics – provide consultation on research methodology, study design, protocol and statistical analysis.
- Support and facilitate research activities in the hospital through the provision of :
- Database of clinicians interested in research – by interest areas, track record on Industry Sponsored Research (ISR).
- Technical support such as statistical analysis.
- Administrative support such as providing research assistants and study coordinators.
- Facility support such as statistical software, study equipment for ISR and Investigator Initiated Research (IIR) such as fridges, centrifuge machines, etc.
- Assist in the process of registration for National Medical Research Register (NMRR) and application of grants for research.
- Participate in clinical trials and other research initiated or coordinated by the ICR.
- Establish collaboration with local, regional and international research organizations in the pursuit for excellence in clinical research in the country.
Meet Our Collaborators
CRC Hospital Ampang, Selangor
Email: ampangcrc@gmail.com