Clinical Research Centre
Hospital Miri
Sarawak
WhoWe Are
The Clinical Research Centre Hospital Miri (CRC Hospital Miri) was officially launched by YB Datuk Lee Kim Shin, Sarawak Assistant Minister of Communication & Sports, on February 10, 2012, with Dr. Goh Pik Pin (Director of National CRC), Dr. Chin Zin Hing (Deputy Director JKNS), and Dr. Jack Wong Siew Yu (Director of Hospital Miri) in attendance. Its vision is to become a leading clinical research institution in Asia, and its mission is to improve patient health outcomes through ethical and high-quality clinical research. The institution aims to strengthen research capacity by developing data resources and providing training, serve as a resource center for clinical research in Miri, promote contract research among clinicians through feasibility assessments, and support researchers by offering research services and facilities.
OurTeam
InvestigatorsHighlight

Head of CRC, Hematologist Hospital Miri, Sarawak
Email: yongkarying@gmail.com
MRCP (UK), DRC Path Hematology (UK), Fellowship in Clinical Hematology (Malaysia & Singapore)
Areas of interest: Malignant Hematology
Awards and achievements:
- Multiple fellowships in Clinical Hematology from Malaysia
- Multiple fellowships in Clinical Hematology from Singapore
- Recognition for research work through publications in esteemed journals
- Prominent speaker at various national and international conferences
- Contributor to both oral and poster presentations at conferences
Dr. Yong Kar Ying is a dedicated hematologist currently serving at Hospital Miri. Her academic journey began in Bintulu, Sarawak, where she attended SRB St Anthony for primary education and SMK Bandar Bintulu for secondary education. Dr. Yong pursued her tertiary education at the International Medical University (IMU), graduating in 2008. She further specialized in internal medicine, obtaining her MRCP (UK) in 2013, followed by a subspecialty in hematology in 2021. Dr. Yong began her medical career as a house officer at Sarawak General Hospital in Kuching from 2008 to 2010, where she gained comprehensive experience across various medical disciplines. She then served as a Medical Officer in Klinik Kesihatan Tatau, Bintulu, and later returned to Sarawak General Hospital, Kuching. From 2014 to 2018, Dr. Yong worked as a Clinical Physician at Miri General Hospital, where she also provided her expertise to district hospitals in Lawas, Marudi, and Limbang. Her hematology training took her to prominent institutions, including Sarawak General Hospital, Hospital Ampang, and the National University Hospital in Singapore. Since January 2022, Dr. Yong has been an integral part of the medical team at Hospital Miri, contributing her expertise in hematology to the local community
Research Highlights
Hematology
Recent Publications
Effectiveness of Omalizumab in Severe Allergic Asthma: Case Series in Sarawak
Prevalence Of Antibiotic Resistance In Culture Positive Burkholderia Pseudomallei Cases Presented To Miri General Hospital Between January 2011 And May 2015: A Cross Sectional Study
Project Highlights
A Deep Learning Time Frequency Decomposition of Gait and Photoplethysmography Features for Continuous Blood Glucose Estimation for Non-Invasive Monitoring of Diabetes Mellitus
This project aims to develop and systematically evaluate predictive models that accurately estimate blood glucose levels (BGL) using gait and photoplethysmography (PPG) data collected from non-invasive wearable sensors. The study involves collecting comprehensive and synchronized gait, PPG, and BGL data from a diverse cohort over six months. Advanced machine learning and deep learning techniques will be employed to analyze this data, identifying patterns that correlate with BGL changes. The predictive models will be rigorously validated against continuous glucose monitoring (CGM) device readings to ensure accuracy and reliability. The findings will contribute to the broader field of non-invasive health monitoring, offering insights into the effectiveness of using gait and PPG data for BGL estimation.

An Observational Study on the Efficacy of Non-Invasive Wearable Sensors for Lower Limb Motion Monitoring to Assist Physiotherapy Assessment
This study aims to evaluate the feasibility of utilizing non-invasive wearable sensors to quantitatively measure lower limb motions, enhancing physiotherapy assessments. The primary objective is to assess how parameters such as angle range of motion and walking speed can aid physiotherapists and doctors in distinguishing between normal and abnormal gait patterns. Additionally, the study explores the potential for wearable sensors to enable self-monitoring of lower limb motions by individuals, particularly in home or rural settings, without direct supervision from healthcare professionals.

| NMRR-21-02117-CI9 | Knowledge, Attitude and Practice Regarding Clinical Research Among Researchers from Government Hospitals and Institute for Clinical Research, Malaysia |
| NMRR-22-02260-DHZ |
Attitudes and Beliefs to Use of Medical Cannabis among Malaysian’s Doctors |
| NMRR-24-01613-GMS | An Observational Study on the Efficacy of Non-Invasive Wearable Sensors for Lower Limb Motion Monitoring to Assist Physiotherapy Assessment |
| NMRR-24-00937-2WM | Effective Shared Decision-Making: Charting the Course to Lower Cholesterol by Cutting Coffee Consumption |
| NMRR-24-01109-W8P | Epidemiology and Risk Factors of Cancer Associated Thrombosis Among Asian Population - A Single Centre Experience |
| NMRR-24-02221-VVM | Overcoming Odds: Unprecedented Response in Chronic Myeloid Leukaemia with Pelvic Myeloid Sarcoma – Harnessing Radiotherapy and Tyrosine Kinase Inhibitor for a Triumph |
| RSCH-24-01765-BUZ | Radiotherapy as a Primary Treatment Modality for Solitary Penile Plamacytoma: A Case Report |
| RSCH-24-02890-LUU | Demographic Characteristics and Outcome of PNH Patients in Sarawak, Malaysia |
| RSCH-24-02863-0PS | Evaluation of Venous Thromboembolism in Adult Lymphoma using Sarawak Lymphoma Thrombosis Score (SLOTS): A Multicenter Retrospective Study in Sarawak |
| RSCH-24-02504-F2X | A Deep Learning Time Frequency Decomposition of Gait and Photoplethysmography Feature for Continuous Blood Glucose Estimation for Non-Invasive Monitoring of Diabetes Mellitus |
| NMRR-24-00982-UTH | Radiotherapy as A Primary Treatment Modality for Solitary Penile Plasmacytoma: Case Report and Literature Review |
| NA | A Systematic Review and Meta-analysis of Factor Associated with Resilience in Older People |
| NA | Profiling of biomarker and Characterisation of Circulating miRNAs in Lymphoma Patients |
| NMRR-23-00525-RKY |
A Randomized, Double-Blind, Placebo-Controlled Multicenter Study to Evaluate the Effect of Inclisiran on Preventing Major Adverse Cardiovascular Events in High-Risk Primary Prevention Patients (VICTORION-1 PREVENT) |
| NMRR-23-00644-HN1 | A Phase 3, Randomized, Double-Blind, Placebo-controlled, Event-driven Study to Demonstrate the Efficacy and Safety of Milvexian, an Oral Factor XIa Inhibitor, After a Recent Acute Coronary Syndrome |
| NMRR-23-01396-SBI | A Randomized, Double-Blind, Parallel Group, Multicenter 24 Week Study to Assess the Efficacy and Safety of Budesonide and Formoterol Fumarate Metered Dose Inhaler Relative to Budesonide Metered Dose Inhaler and Open-Label Symbicort® Turbuhaler® in Participants with Inadequately Controlled Asthma (VATHOS) |
| NMRR-23-01608-6GH | A Single Arm Study Investigating the Glycaemic Control and Safety of Adding Semaglutide to Insulin Icodec in Participants with Type 2 Diabetes Qualifying for Treatment Intensification |
| NMRR-23-01803-SNW | A Randomized, Participant- and Investigator-Blinded, Placebo-Controlled, Parallel Group Study to Assess the Efficacy, Safety and Pharmacokinetics of EYU688 in Patients with Dengue Fever |
| NMRR-23-01871-OBV | A Randomized, Double-Blind, Parallel Group, Multicenter 12 Week Study to Assess the Efficacy and Safety of Budesonide and Formoterol Fumarate Metered Dose Inhaler Relative to Budesonide Metered Dose Inhaler in Participants with Inadequately Controlled Asthma (LITHOS) |
| NMRR-23-02718-WNX | A Randomised, Double-Blind, Placebo-Controlled, Parallel Group Study to Assess the Efficacy and Safety of Baxdrostat in Participants with Uncontrolled Hypertension on Two or More Medications including Participants with Resistant Hypertension |
| NMRR-23-02850-UTZ | A Phase III, Multicentre, Randomised, Double-blind, Chronic-dosing, Parallel-group, Placebo-controlled Study to Evaluate the Efficacy and Safety of Tozorakimab in Participants with Symptomatic Chronic Obstructive Pulmonary Disease (COPD) with a History of COPD Exacerbations (MIRANDA) |
| NMRR-23-03025-C7C | A Randomized, Multi-regional, Double-blind, Double-dummy Parallel group, Placebo and Allopurinol-controlled Phase 3 Study to Assess the Efficacy and Safety of Tigulixostat in Gout Patients with Hyperuricemia |
| NMRR-23-03081-6UF | Efficacy and Safety of First Line Use of Oral Semaglutide 25 mg or 50 mg Once Daily Versus Empagliflozin 25 mg or Versus Metformin 2000 mg in Newly Diagnosed Treatment Naïve Patients with Type 2 Diabetes |
| NMRR-23-03227-9DZ | The Cardiovascular Safety and Efficacy of Cagrilintide 2.4 mg s.c. in Combination with Semaglutide 2.4 mg s.c. (CagriSema 2.4 mg/2.4 mg s.c.) Once-Weekly in Participants with Established Cardiovascular Disease |
| NMRR-24-00421-VYY | A Randomised, Double-Blind, Placebo-Controlled, Parallel Group Study to Assess the Effect of Baxdrostat on Ambulatory Blood Pressure in Participants with Resistant Hypertension |
List of Publications associated with CRC Hospital Miri
- Effectiveness of a Pharmacist-Led, Community Group-Based Education Programme in Enhancing Diabetes Management: A Multicentre Randomised Control Trial
- Ahmad K, Anchah L, Ting CY, Lim SE
- Long-Term Clinical Outcomes of Reduced Dose of Emicizumab in Haemophilia A with Inhibitors: A Real World Experience in Sarawak, Malaysia.
- Kar Ying Yong, Ching Tiong Ko, Andy Sing Ong Tang , Lee Ping Chew
- Demographics and Outcome of Patients with Congenital Bleeding Disorder in Sarawak.
- Andy Tang Sing Ong, Leong Tze Shin, Yong Kar Ying, Grace Lee Wan Chieng, Ko Ching Tiong, Chew Lee Ping
- Clinical Presentation and Management of Immune Thrombotic Thrombocytopenic Purpura (iTTP) in Borneo Populations – A Case Series.
- W.J. LAU, K.Y. YONG, T.S. LEONG , L.P. CHEW
Services at CRC Hospital Miri
CRC Hospital Miri provides training through workshops and consultations:
-
Research related Training
-
Supporting IIR & ISR
-
Introduction to Clinical Research Workshop
-
Manuscript Writing Workshop
-
Good Clinical Practice Workshop
-
Basic SPSS Workshop
-
Intermediate SPSS Workshop
-
Research Methodology (Intermediate)
-
Qualitative Research Workshop
Meet Our Collaborators

Upcoming Activities


Recent Activities

Investigator Meeting – WA43966
- 30th January – 1st February 2024
- Investigator meeting in South Korea, Seoul.
- Protocol: A Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study To Evaluate the Efficacy and Safety of RO7434656, An Antisense Inhibitor of Complement Factor B, In Patients with Primary IgA Nephropathy at High Risk of Progression.

Beginner’s Guide To Clinical Research Workshop
- 29th Feb 2024 – 1st March 2024
- Transforming ideas into research: refine concepts, frame a testable hypothesis and establish objectives to drive clinical research question effectively.
- Literature review: develop an effective search strategy to suit research topic, reviewing existing studies and identify gaps.
- Study design: learn about various basic study designs and how to select the most appropriate study design.
- Basic statistics: explore the basic concepts of statistics, including different descriptive and inferential statistical tests.

CRC Network Meeting 1/2024
- 4th – 5th March 2024
- Team building session among admin managers from various CRC Network.
- TOR for admin managers and other CRC staff.
- SKU review.
- SRB and funding for article publication.
- Administrative updates for CRC’s and ICR centres.

Healthy Strides Malaysia
- 11th March 2024
- International collaboration for special needs children, whereby our team in Miri are partners with the Healthy Strides Foundation in Perth Australia, in association with Sarawak Oil Palms Foundation – bringing a world leading intervention for children with neurodisabilities.
- Check out the article on the evening in:
1)New Sarawak Tribune
2)We Are United
3)Utusan Borneo
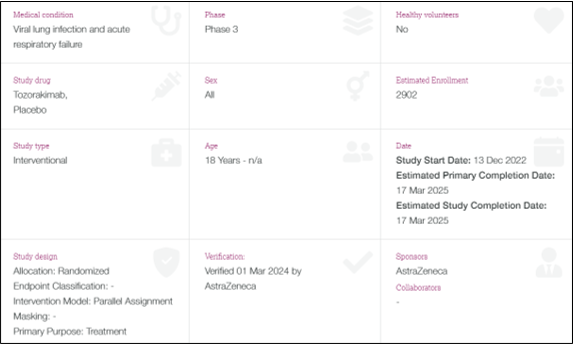
Investigator Site Audit – TILIA
- 19th – 21st March 2024
- Protocol: A Phase III, Multicentre, Randomised, Double-blind, Paralled-group, Placebo-controlled Study to Evaluate the Efficacy and Safety of Tozorakimab in Patients Hospitalised for Viral Lung Infection Requiring Supplemental Oxygen (TILIA)
- Routine investigator site audit, whereby the review of the TILIA study lasted for 3 days.

Investigator Meeting - BAxHTN
- 23rd – 24th March 2024
- Investigator Meeting in Bangkok, Thailand.
- Protocol: A Randomised, Double-Blind, Placebo-Controlled, Parallel Group Study to Assess the Efficacy and Safety of Baxdrostat in Participants with Uncontrolled Hypertension on Two or More Medications including Participants with Resistant Hypertension.
Basic Research Methodology & Biostatistics Workshop 2024
- 18th – 19th April 2024
- In collaboration with JKNS Pharmacy Department.
- Platform: Google Meet (Virtual)
- Objectives: To enable participants to acquire skills using necessary methodology & statistical tools in designing & conducting good quality clinical research.
- To understand and apply knowledge in conducting clinical research.

Good Clinical Practice Workshop & Certified Examination 2024
- 23rd – 25th April 2024
- Venue: Meritz Hotel, Miri
- Objective: To understand the underlying principles of GCP & its specific rules of conduct, providing experience in the keys skills required through simulation in classroom setting, to provide some of the resources required to design & conduct GCP trial and to achieve an overall understanding on how to conduct GCP compliance trial.

CRM CONNECT & NCCR 2024
- 9th – 10th May 2024
- Venue: Nexus @ Connexion Conference & Event Centre
- CRM Trial CONNECT is now on its 3rd year, with new collaborators on board the program, The European Organisation for Research and Treatment of Cancer (EORTC), National Cancer Center Japan (NCC) & National Conference for Clinical Research (NCCR) is joining under the CRM Trial CONNECT umbrella, and together having an impactful program curated that showcases Malaysia’s capabilities, experiences and achievements in clinical research.

ICR Director Site Visit In Conjunction with Training for Trainers: Introduction to Clinical Research
- On 13th May 2024, CRC Miri had the distinct honour of welcoming Datin Dr. Sheamini Sivasampu, Director of the Institute for Clinical Research (ICR), NIH, along with esteemed delegates from the Sarawak State CRC Network. The visit was marked by a productive and engaging discussion on advancing clinical research in the region.
- In conjunction with this visit, "Training for Trainers: Introduction to Clinical Research" workshop is on-going from 14th May to 15th May 2024. This training aims to equip our researchers with the latest methodologies and insights in clinical research.

“I AM AWARE” CAMPAIGN
- On May 21, 2024, Miri Hospital hosted the “I Am Aware” campaign, aimed at raising awareness of clinical trials among patients and the general public. This campaign offer crucial information on clinical research, helping to educate and inform. The Clinical Research Centre (CRC) and Clinical Research Malaysia (CRM) are key partners in this initiative, ensuring a thorough and effective outreach.
Intermediate Biostatistics & Data Analysis Method Workshop
- On 19th – 20th June 2024, an Intermediate Biostatistics and Data Analysis Method Virtual Workshop was conducted, gathering an estimated 36 participants. The workshop aimed to enhance participants' skills in intermediate biostatistics and data analysis, covering topics such as multiple regression, ANOVA, logistic regression, survival analysis, and data visualization. It featured a blend of theoretical sessions and practical hands-on exercises using statistical software. Participants engaged in virtual group discussions, collaborative exercises, and real-world applications of biostatistics, culminating in group presentations of their data analysis findings. The workshop concluded with a virtual networking session, with feedback collected to evaluate the workshop's effectiveness.

Qualitative Research Workshop
- On 24th June 2024, a Qualitative Research Virtual Workshop was conducted with estimated 56 participants. The workshop aimed to deepen participants' understanding of qualitative research methodologies, including ethnography, grounded theory, and phenomenology. The event featured interactive sessions on data collection techniques such as interviews and focus groups, as well as data analysis methods like coding and thematic analysis. Participants engaged in virtual breakout sessions for hands-on practice and group discussions to apply the concepts learned. The workshop concluded with a Q&A session, and a virtual networking opportunity. Feedback was collected to assess the workshop's success and areas for improvement.

Benchmarking Visit to CRC SGH
- 28th June 2024
- This programme is proposed to observe the facilities provided at CRC SGH and bring about it into CRC Hospital Miri so that the centre could provide excellent clinical research service with utmost efficiency with the latest technology in its method and equipment in future. This program is also done to be able to improve the clinical research procedures by gaining some additional knowledge through a sharing session from the clinical research staff of SGH to meet the needs of patients swiftly and smoothly. In addition, the previous Head of Department of CRC Miri Hospital, Dr Jack Wong has “an ultimate goal” to bring and initiate Phase 1 studies and BA-BE studies in Hospital Miri in near future.
CRC Hospital Miri, Sarawak
Email: mirihospitalcrc@gmail.com









