Clinical Research Centre
Hospital Sultanah Bahiyah
Alor Setar, Kedah
WhoWe Are
Established in 2006, Clinical Research Centre Hospital Sultanah Bahiyah (CRC HSB) is part of a thriving network of CRCs and function as a vital bridge between the Ministry of Health, research institutions and industry partners. By fostering collaboration on crucial research projects, we empower researchers to develop solutions that directly address the needs of the underserved communities. Our unwavering commitment has demonstrably reshaped the healthcare landscape, bringing us closer to a future where quality healthcare is within reach for all.
OurTeam
InvestigatorsHighlight

Head of CRC, Hospital Sultanah Bahiyah, Alor Setar
Email: azrisuan@crc.moh.gov.my
LinkedIn: www.linkedin.com/in/mohd-azri-mohd-suan-312196249
MBBS (Malaya), MSc. Epidemiology & Biostatistics (UPM)
Areas of interest: Clinical trial, Gastroenterology & Hepatology, Public Health, Health System Research, Research Methods & Statistics
Awards and achievements:
- Attending summer course in health research and statistics at Erasmus Medical Center, Rotterdam, The Netherland under Latihan Dalam Perkhidmatan, 2016.
- Recipient of Reckitt Pediatric Global Resident Scholarship Award 2024 for study on 'Types of Early Enteral Feeding for Premature Infants and Its Impact’. Qun Yuan Goh, Eric Boon Kuang Ang, Mohd Azri Mohd Suan, Shahrul Aiman Soelar. April 2024.
I am a Medical Officer and Researcher with more than 10 years of experience in the health research. My passion lies in bridging the gap between research and clinical practice to improve patient outcomes. At the Clinical Research Center, Hospital Sultanah Bahiyah, I lead a team dedicated to conducting high-quality clinical trials and promoting evidence-based healthcare practices. I possess strong research skills in areas like clinical trials, research methodology, health systems research, and medical statistics. Additionally, I have been involved in numerous Industry-sponsored research (ISR) and Investigator-initiated Trials (IIT). My research efforts have resulted in the publication of many articles in various journals.
Research Highlights
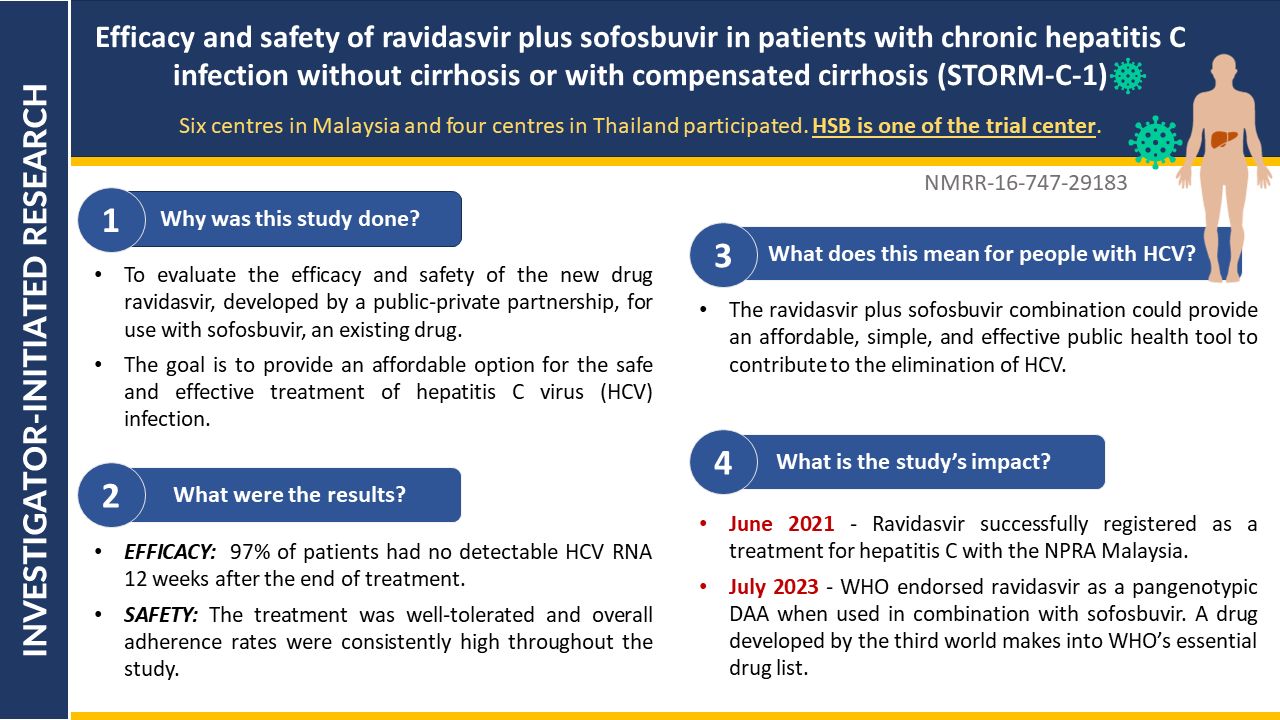
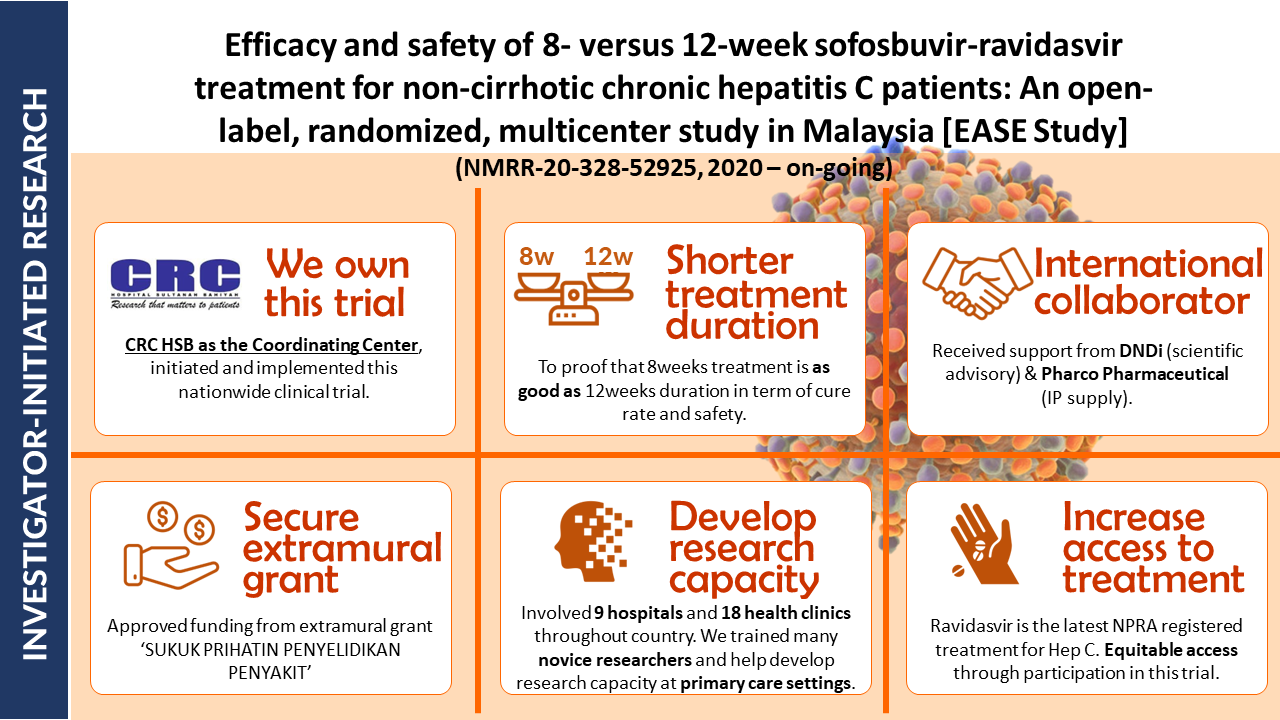
- Efficacy and Safety of 8- versus 12-week Treatment with sofosbuvir-Ravidasvir for Chronic, Non-Cirrhotic, Hepatitis C: Interim Analysis of a Randomized Controlled Trial in Malaysia
- Shortening treatment: This study investigates whether reducing treatment duration from 12 to 8 weeks with sofosbuvir-ravidasvir is equally effective in curing chronic hepatitis C without cirrhosis in Malaysian patients.
- Potential impact: Final results will be out soon. If confirmed, these findings could significantly impact hepatitis C treatment guidelines, potentially reducing treatment costs and increasing treatment accessibility.
- Feasibility, Usability and Cost of Conventional Versus Single-Use Biodegradable Meal Trays: A Pilot Study in Hospital Sultanah Bahiyah, Alor Setar
- Innovative solution: This study explored the potential of single-use biodegradable meal trays made from paddy straw as a sustainable alternative to conventional meal trays in a Malaysian hospital.
- Policy implications: The findings of this study provide compelling evidence for policymakers and hospital administrators to consider adopting biodegradable meal trays as a sustainable and cost-effective solution.
-
Implementing a new Prison-based Test-and-Treat Model for Enhancing Hepatitis C Care in Kedah, Malaysia
-
Addressing a gap: This study fills a critical knowledge gap by evaluating the feasibility of a prison-based test-and-treat model for hepatitis C (HCV).
-
Policy implications: The findings support the broader adoption of prison-based test-and-treat models as a crucial component of HCV elimination strategies.
-
Recent Publications
Project Highlights
| NMRR-20-328-52925 |
Efficacy and Safety of 8-versus 12-week Sofosbuvir-Ravidasvir Treatment for Non-Cirrhotic Chronic Hepatitis C Patients: An Open-Label, Randomized, Multicenter Study in Malaysia
|
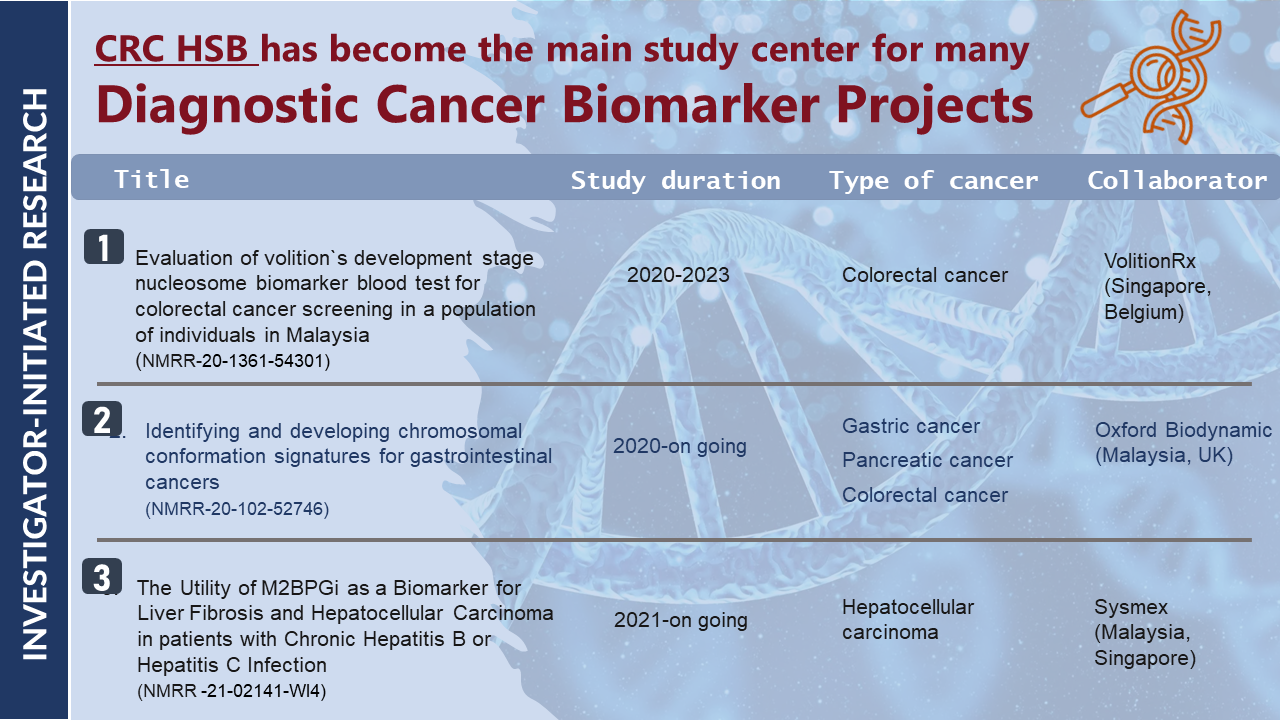
| NMRR-21-02141-WI4 |
The Utility of M2BPGi as a Biomarker for Liver Fibrosis and Hepatocellular Carcinoma in Patients with Chronic Hepatitis B or Hepatitis C Infection
|
| NMRR-21-80-57920 |
Cost Analysis on Locally Manufactured Single-Use Biodegradable Hospital / Patient-Care Utensils at Hospital Sultanah Bahiyah
|
| NMRR-23-00196-WJ0 |
Investigation of The Microplastics Pathways between The Environment and The Human Colon as A Mechanism to Explain the Potential Risk of Colon Cancer
|
| NMRR-22-00251-0XV |
Impact of Stoma Formation on The Quality of Life Among Colorectal Cancer Patients in Malaysia: A Qualitative Study
|
| RSCH-22-03689-Z3V |
Measures to Guide the Distribution of Internal Medicine Physician
|
| NMRR-16-1987-33040 |
Understanding of Childhood Immunisation and Reasons for Refusal among the Malaysian Parents: A Qualitative Study
|
| NMRR-22-02970-KA2 |
Understanding Parental Decision-Making in the Enrollment of Children in Clinical Trials: A Qualitative Study
|
| NMRR-22-02260-DHZ |
Attitudes and Beliefs to Use of Medical Cannabis among Malaysian’s Doctors
|
| NMRR-22-00003-6NQ |
Effect of High Dose Intravenous Vitamin C as an Adjunct in the Treatment of Patients with Severe Pneumonia in Intensive Care Unit: A Multi-Center, Double-Blinded, Two-Arm, Placebo-Controlled, Randomized Trial
|
| NMRR-15-596-25330 |
Cost Efficiency Analysis of Malaysian Hospitals
|
| NMRR-22-02345-5QG |
Cholangiocarcinoma Incidence, Trends, Risk Factor and Survival Rate in Northern Region of Malaysia: Analysis of 10 Years Data (2012-2022)
|
| RSCH-23-00015-TOP |
Economic Evaluation of Sofosbuvir-Ravidasvir Treatment for Non-Cirrhotic Chronic Hepatitis C Patients: An Extension of EASE Trial
|
| NMRR-20-2288-56852 |
10-year Prevalence of Non Syndromic Cleft Lip, Cleft Palate and Cleft Lip and Palate among Children Born in a Tertiary Hospital, Malaysia: A Retrospective Study
|
| NMRR-22-02371-5NY |
Public Perception on Generational End Game (GEG)
|
| RSCH-22-01041-7RV |
Barriers to Colorectal Cancer Screening in Malaysia: A Systematic Review
|
| NMRR-22-02802-JMO |
Development of Validated Predictive Pharmacodynamic Models of Initial Warfarin Dose: A Mixed-Method Study
|
| NMRR-19-1188-47227 |
Investigation of Urinary MicroRNA as Potential Biomarker for Colorectal Cancer Detection
|
| NMRR-19-1013-47474 |
The Evaluation of 13 Years Behavioural Modification in Smoking Cessation Programme by Health Educators at Hospital Sultanah Bahiyah, Alor Setar
|
| NMRR-18-3157-44781 |
Trending of Comprehensive Dental Treatment Under General Anesthasia in 8 Years at Paediatric Dental Clinic, Hospital Sultanah Bahiyah, Alor Setar
|
| NMRR-23-00545-UTT |
Clinical Outcome of COVID-19 Patient in Intensive Care Unit Hospital Sultan Abdul Halim in 2021
|
| NMRR-21-174-57969 |
Molar Tooth Survival of Periodontal Patients Following Periodontal Therapies in Klinik Pakar Periodontik Kota Setar
|
| NMRR-20-1217-55489 |
Facial Anthropometry Survey Among Malaysian for The Development of Bivariate and Principal Component Analysis Facial Panel
|
| NMRR-20-2807-57057 |
Phase II/III Randomized, Controlled Clinical Study of AlloStim® VS. Physician's Choice in Asian Subjects with Advanced Hepatocellular Carcinoma
|
| NMRR-20-102-52746 |
Identifying and Developing Chromosomal Conformation Signatures for Gastrointestinal Cancers
|
| NMRR-23-00652-I4A |
Identifying and Developing Chromosomal Conformation Signatures for Early Screening and Diagnosis of Colorectal Cancer
|
| NMRR-20-1140-53693 |
Treatment of CVD with Low dose Rivaroxaban in Advanced CKD (TRACK)
|
| NMRR-19-3823-52045 |
Randomised Evaluation of Sodium Dialysate Levels on Vascular Events (RESOLVE)
|
| NMRR-22-00178-HCX |
A Phase 3, Randomized, Double-Blind, Active-Control Study of Pelabresib (CPI-0610) and Ruxolitinib vs Placebo and Ruxolitinib in JAKi Treatment Naïve MF Patients (MANIFEST-2)
|
| NMRR-22-02468-BUD |
A Phase IIIb, Multi-Center, Open-Label, Randomized Study of Tolerability and Efficacy of Oral Asciminib versus Nilotinib in Patients with Newly Diagnosed Philadelphia Chromosome Positive Chronic Myelogenous Leukemia in Chronic Phase (CABL001J12302)
|
| NMRR-22-02956-NIS |
A Phase 2B Randomized, Double-Blind, Active and Placebo-Controlled, Parallel-Group, Multicenter Study to Evaluate the Efficacy and Safety of Induction and Maintenance Combination Therapy with Guselkumab and Golimumab in Participants with Moderately to Severely Active Crohn’s Disease (DUET-CD)
|
| NMRR-17-1636-36783 |
A Randomized, Multicenter, Double-Blind, Parallel-Group, Placebo-Controlled Study to Investigate the Efficacy and Safety of Canagliflozin in Children and Adolescennts (≥10 to <18 years) with Type 2 Diabetes Mellitus (DIA)
|
| NMRR-19-1258-47901 |
Semaglutide Cardiovascular Outcomes Trial in Patient with Type 2 DM (SOUL)
|
| NMRR-19-770-47233 |
A Phase 3b, Open Label, Single-Arm, Rollover Study to Evaluate Long-Term Safety in Subjects who have Participated in Other Luspatercept (ACE-536) Clinical Trials (ACE-536-LTFU-001)
|
| NMRR-21-01964-JHU |
A Phase 3, Double-Blind, Randomised, Placebo-Controlled, Multicenter Study Evaluating the Efficacy and Safety of Mitapivat in Subject with Non-Tranfusion-Dependent Alpha- or Beta-Thalasemia (ENERGIZE)
|
| NMRR-21-01965-4JL |
A Phase 3, Double-Blind, Randomised, Placebo-Controlled, Multicenter Study Evaluating the Efficacy and Safety of Mitapivat in Subjects with Tranfusion-Dependent Alpha- or Beta-Thalasemia (ENERGIZE-T)
|
Scope of Training and Services
Consultation:
- Protocol Development
- Trial Initiation
- Statistical Analysis
- Clinical Research Planning and Coordination
- Project Management
- Study Report/Manuscript Writing
- Grant Application
- Trial Monitoring
- Submission for Journal Publication
- NMRR Registration & MREC Approval
- Clinical Data Management
Our Research Consultation Clinic: Tue & Wed (8am - 5pm)

Meet Our Collaborators







Kindly visit CRC Kedah for further info on upcoming activities
CRC Hospital Sultanah Bahiyah, Alor Setar, Kedah
Level 2,
KM 6 Jalan Langgar,
05460 Alor Setar, Kedah
Email: crc.hsbkedah@gmail.com