Clinical Research Centre
Hospital Seri Manjung
Perak
WhoWe Are
Clinical Research Centre Hospital Seri Manjung (CRC HSM) was established since 2011, with the collaboration of Clinical Research Malaysia. We aim to improve patients’ health through ethical and quality clinical research. We manage Investigator Initiated Research (IIR) as well as Industry-Sponsored Research (ISR). The availability of medical facilities and investigators at the site contribute to the increasing number of clinical research in Hospital Seri Manjung. In addition, there are ongoing research consultations throughout the year to assist our colleagues in conducting research, such as National Medical Research Register (NMRR) registration, protocol development, literature search, statistical analysis, manuscript write-up and assistance in submission for publication of research papers. We also provide research-related trainings such as Good Clinical Practice (GCP) Workshop, Introduction to Clinical Research (ICR) Workshop, Basic Biostatistics Workshop, Proposal Writing Workshop, Literature Search and Referencing Workshop, Scientific Writing Workshop, NMRR Registration Workshop, etc.
OurTeam
InvestigatorsHighlight

Head of CRC, Consultant Paediatrician, Hospital Seri Manjung (HSM
Email: ngashihhang@gmail.com
MBBS, MRCPCH
Areas of interest: Paediatric Asthma, Diabetes, Neonatology
Awards and achievements:
- Excellent Service Award, Ministry of Health Malaysia - 2006, 2011
Dr. Nga, who holds an MBBS and MRCPCH in Paediatrics, earned his GCP certification in 2006 and soon began his first Industry Sponsored Research (ISR). His subsequent ISRs have focused on paediatric asthma, Type 2 diabetes, and RSV infections. He is currently the head of the Clinical Research Centre at Hospital Seri Manjung, a 305-bed minor specialist hospital offering services in various fields including Internal Medicine, Obstetrics, Surgery, Paediatrics, and more. Dr. Nga has conducted local audits on topics such as neonatal intravenous cannulation, patient falls in paediatric wards, sexual abuse in suburban areas, and iron overload in thalassemia. He also published a case report on Refractory Kawasaki Disease in the Medical Journal of Malaysia.
Recent Publications
Research Work Involvement
- Oral Diosmectite reduces stool output and diarrhoea duration in children with acute watery diarrhoea
- Safety of Adding Salmeterol to Fluticasone Propionate in Children with Asthma (as Country Coordinating PI)
- T2NOW: A 26-Week, Multicenter, Randomized, Placebo-Controlled, Double-Blind, Parallel Group, Phase 3 Trial with a 26-Week Safety Extension Period Evaluating the Safety and Efficacy of Dapagliflozin 5 and 10 mg, and Saxagliptin 2.5 and 5 mg in Pediatric Patients with Type 2 Diabetes Mellitus Who Are Between 10 and Below 18 Years of Age
- ReViral: A Phase 2 Open-Label Study in Infants with REspiratory Syncytial VIRus Lower RespirAtory Tract Infection, Followed by a DoubLe-blind, Placebo controlled Part, to Evaluate the Safety, Tolerability, Pharmacokinetics and Antiviral Effect of RV521 (REVIRAL 1)
- MK 1654: A Phase 2b/3 Double-Blind, Randomized, Placebo-Controlled Study to Evaluate the Efficacy and Safety of MK-1654 in Healthy Pre-Term and Full-Term Infants
- A randomized, double-blind, placebo-controlled, parallel-group study to assess the efficacy, safety, and tolerability of dexpramipexole administered orally for 52 weeks in participants with severe eosinophilic asthma (EXHALE-2)
- An Interventional, Phase 1b, Randomized, Double-Blind, Placebo-controlled , Multi-center, Dose-finding Study To Evaluate Safety, Tolerability and Pharmacokinetics Of SISUNATOVIR In Pediatric Participants up to age 60 months with Respiratory SyncitialVirus (RSV) Lower Respiratory Tract Infection (LRTI)
Project Highlights
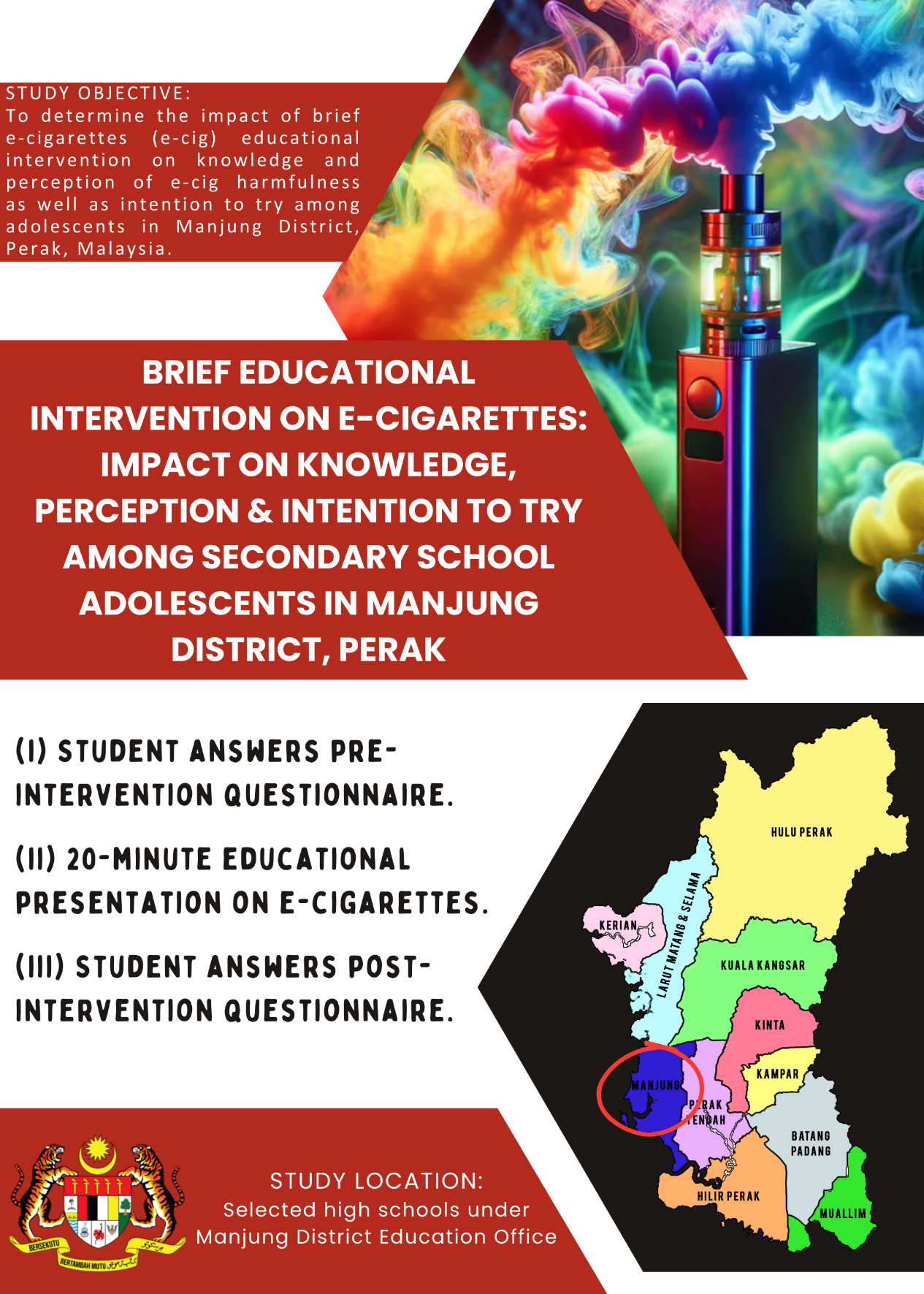
- This is a quasi-experimental study which will be conducted among students at the selected high schools under Manjung District Education Office, Perak.
- This study aims to determine the impact of brief e-cigarettes (e-cig) educational intervention on the knowledge and perception of e-cig harmfulness as well as intention to try among adolescents in Manjung District, Perak, Malaysia.
- This is a cross-sectional study conducted among caretakers of paediatric patients who present at the pharmacy counter of health clinics under Kampar District Health Office.
- This study aims to determine the extent of knowledge and practice of caretakers of paediatric patients on the storage of oral liquid medications.

- This is a retrospective cohort study conducted at Hospital Tengku Ampuan Rahimah, Klang involving retrospective review of hospitalized maternal cases with suspected pulmonary embolism (PE) who had undergone Computed Tomography Pulmonary Angiogram (CTPA) examination.
- This study aims to study the prevalence and clinical features of PE during pregnancy and puerperium.

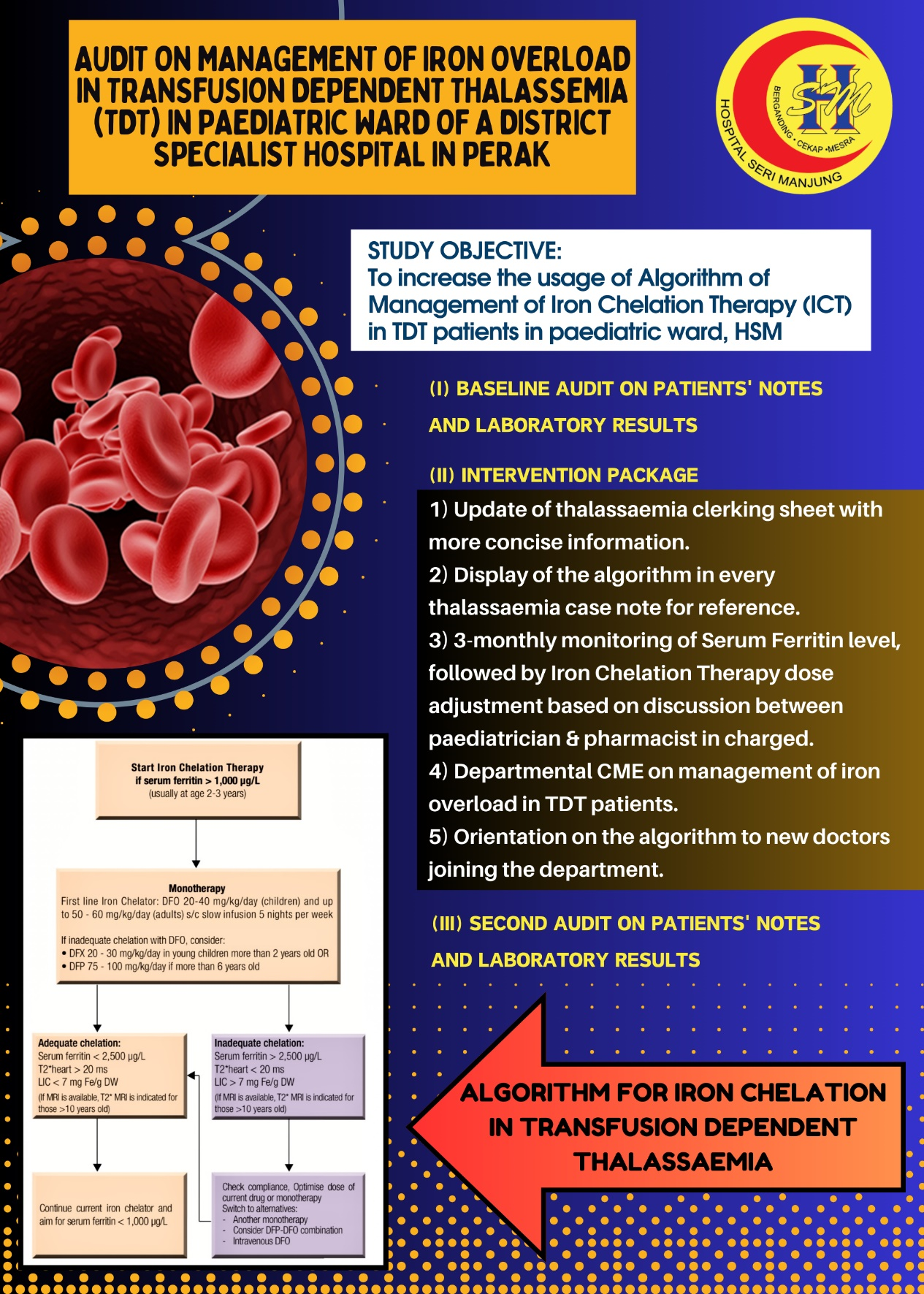
- This project involves two audits on the case notes of Transfusion Dependent Thalassemia (TDT) patients requiring regular blood transfusion in paediatric ward of Hospital Seri Manjung (HSM).
- It aims to increase the usage of Algorithm of Management of Iron Chelation Therapy (ICT) in TDT patients in paediatric ward, HSM.
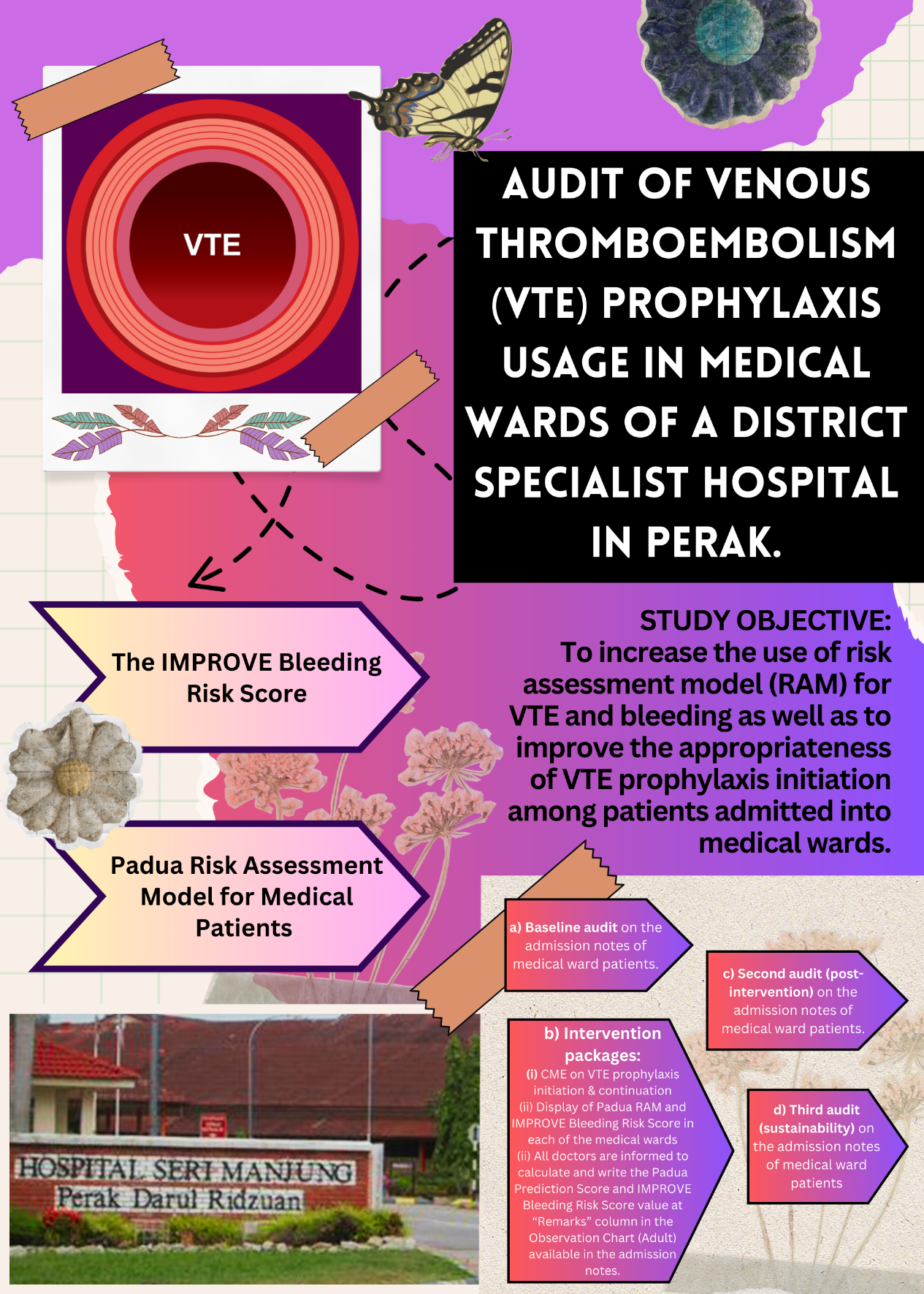
- This project involves three audits on the admission notes of patients hospitalized in medical wards of Hospital Seri Manjung (HSM).
- It aims to increase the use of risk assessment model for Venous Thromboembolism (VTE) and bleeding as well as to improve the appropriateness of VTE prophylaxis initiation.
| Brief Educational Intervention on E-Cigarettes: Impact on Knowledge, Perception & Intention to Try among Secondary School Adolescents in Manjung District, Perak | |
| Aiming for Better Clinical Documentation by the Emergency & Trauma Department Team: A Quality Improvement Project | |
| Diagnosing Hypertriglyceridemic Acute Pancreatitis (HTGAP) with Urine Amylase: Case Report | |
| Assessment of Knowledge and Practice of Caretakers of Paediatric Patients on Storage of Oral Liquid Medications: A Multi-Centre Study | |
| Prevalence and Clinical Features of Pulmonary Embolism in Pregnancy and Puerperium: A Tertiary Hospital Report | |
| Audit on Management of Iron Overload in Transfusion Dependent Thalassemia (TDT) in Paediatric Ward of a District Specialist Hospital in Perak | |
| The Evaluation of Lipid Modifying Agents Prescribing Trend from 2015 to 2020 in Manjung District Perak : A Clinical Audit | |
| Cardiac Cephalgia - Headache of the Heart: Case Report | |
| Audit of Venous Thromboembolism (VTE) Prophylaxis Usage in Medical Wards of a District Specialist Hospital in Perak | |
| Attitudes and Beliefs to Use of Medical Cannabis among Malaysian's Doctors | |
| The Rate of Blood Culture Contamination and Common Organisms Isolated in Malaysian Public Hospitals | |
| A Descriptive Study of Neonatal Encephalopathy (NE) in the Neonatal Intensive Care Unit of Seri Manjung Hospital in Perak, A Minor Specialist Hospital in Malaysia | |
| Two Cases of Lethal Junctional Haemorrhage: A District General Hospital Experience | |
| COVID-19 Related Occupational Stress Perceived by Medical Officers in a Hospital in Northern Malaysia | |
| Factors Associated with Surgical Site Infection Post Appendectomy in a Tertiary District Hospital: A Two-Year Analysis | |
| Private vs Public Pharmacist: Patients' Experience on Medication Counselling | |
| Knowledge, Attitude and Practice of Pregnant Women Regarding Anaemia during Antenatal Visit at Kampar Health Clinic | |
| Sleep Disturbance in School-aged Children with Autistic Spectrum Disorder: Prevalence and Effect to Parental Stress in Penang, Malaysia | |
| Moral Distress among Healthcare Professionals in Malaysia during the COVID-19 Pandemic | |
| Are Malaysian Children Aware of Their Rights? |
| D4325C00010 (ZENITH High Proteinuria) A Phase III, Randomised, Multicentre, Double-blind Study to Evaluate the Efficacy, Safety, and Tolerability of Zibotentan/ Dapagliflozin Compared to Dapagliflozin Alone in Participants with Chronic Kidney Disease and High Proteinuria |
|
| WA43966 (IMAGINATION) A Phase III, Multicenter, Randomized, Double Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety Of RO7434656, an Antisense Inhibitor of Complement Factor B, in Patients with Primary IgA Nephropathy at High Risk of Progression |
|
| 70033093AFL3002 (LIBREXIA AF) A Phase 3, Randomized, Double-Blind, Double-Dummy, Parallel Group, Active-Controlled Study to Evaluate the Efficacy and Safety of Milvexian, an Oral Factor XIa Inhibitor, versus Apixaban in Participants with Atrial Fibrillation |
|
| NN9838-4942 (REDEFINE 3) The Cardiovascular Safety and Efficacy of Cagrilintide 2.4 mg s.c. in Combination with Semaglutide 2.4 mg s.c. (CagriSema 2.4 mg/2.4 mg s.c.) Once-weekly in Participants with Established Cardiovascular Disease |
|
| LG-GDCL010 (EURELIA 2) A Randomized, Multi-regional, Double-blind, Double-dummy Parallel-group, Placebo and Allopurinol-controlled Phase 3 Study to Assess the Efficacy and Safety of Tigulixostat in Gout Patients with Hyperuricemia |
|
| D6970C00002 (BAXHTN) A Randomised, Double-Blind, Placebo-Controlled, Parallel Group Study to Assess the Efficacy and Safety of Baxdrostat in Participants with Uncontrolled Hypertension on Two or More Medications including Participants with Resistant Hypertension |
|
| CKJX839D12302 (VICTORION-1 PREVENT) A Randomized, Double-blind, Placebo-controlled Multicenter Study to Evaluate the Effect of Inclisiran on Preventing Major Adverse Cardiovascular Events in High-risk Primary Prevention Patients |
|
| D9487C00001 (DIALIZE-Outcomes) An International, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Effect of Sodium Zirconium Cyclosilicate on Arrhythmia-Related Cardiovascular Outcomes in Participants on Chronic Hemodialysis with Recurrent Hyperkalemia |
|
| EX6018-4758 (ZEUS) Effects of Ziltivekimab versus Placebo on Cardiovascular Outcomes in Participants with Established Atherosclerotic Cardiovascular Disease, Chronic Kidney Disease and Systemic Inflammation |
|
| D9488C00001 (STABILIZE-CKD) A Phase 3, International, Randomised, Double-blind, Placebo-controlled Study to Evaluate the Effect of Sodium Zirconium Cyclosilicate on Chronic Kidney Disease (CKD) Progression in Participants with CKD and Hyperkalaemia or at Risk of Hyperkalaemia |
|
| NN9838-4609 (REDEFINE 2) Efficacy and Safety of Cagrilintide s.c. 2.4mg in Combination with Semaglutide s.c. 2.4mg (CagriSema s.c. 2.4mg/2.4mg) Once-weekly in Participants with Overweight or Obesity and Type 2 Diabetes |
|
| KBP5074-3-001 (CLARION-CKD) A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Assess the Efficacy and Safety of KBP-5074, a Mineralocorticoid Receptor Antagonist, in Subjects with Uncontrolled Hypertension Who Have Moderate or Severe (Stage 3b/4) Chronic Kidney Disease |
|
| 21177 (FIND-CKD) A Randomized, Double-blind, Placebo-controlled, Parallel-group, Multicenter Phase 3 study to Investigate the Efficacy and Safety of FInerenone, in addition to Standard of Care, on the Progression of Kidney Disease in Patients with Non-Diabetic Chronic Kidney Disease |
List of Publications associated with CRC HSM
- Lost years, mortality burden: the impact of COVID-19 pandemic on premature death due to road traffic accidents in a northern state in Malaysia
- Premaa Supramaniam, Suria Junus, Lina Hashim, Shoen Chuen Chiew, Philip Rajan Devesahayam
- Development of a novel questionnaire to assess knowledge, attitude and practice towards medical disorders in pregnancy among clinicians
- Thai Lun Tan, Shoen Chuen Chiew, Qinglin Lau, Thanusha, Mahmoud Danaee, Manisha
Services at CRC Hospital Seri Manjung
CRC HSM provides training through workshops and consultations:
-
Research consultations:
- Proposal/Protocol development
- Research question/objective hypothesis
- Study design
- Sample size planning
- Sampling technique
- Instrument/questionnaire
- Statistical planning
- Protocol review
Assist with statistical software:
- Data entry/management
- Data analysis
- Statistical writing
- Abstract/report writing
- NMRR assistance
- DG approval for presentation/publication
- Institution approval
Training:
- Good Clinical Practice (GCP) Workshop
- Introduction to Clinical Research (ICR) Workshop
- Basic Biostatistics Workshop
- Proposal Writing Workshop
- Literature Search and Referencing Workshop
Further info on upcoming activities
CRC Hospital Seri Manjung, Perak
Main Phone: +605-689 6600 Ext: 6639