Clinical Research Centre
Hospital Tunku Azizah
Kuala Lumpur
WhoWe Are
The diversity of clinical cases and the substantial amount of educational activities related to Paediatrics, Obstetrics and Gynaecology make Hospital Tunku Azizah Kuala Lumpur (HTA) a dynamic and leading centre in the country. It started as Unit Penyelidikan in March 2019. Clinical Research Centre HTA (CRC HTA) established on 23rd October 2019. This is the 35th in the country to facilitate clinical research activities in MOH. Its vision is to become the hub of clinical research, especially in the fields of Paediatrics, Obstetrics and Gynaecology by driving the culture of research and innovation among the staff in this hospital. CRC HTA's mission is to facilitate the conduct of high impact, translational and ethical clinical research in fields related to Paediatrics and O&G while their objective is to encourage, facilitate and supervise researchers to produce scientifically valid and ethically sound clinical research.
OurTeam
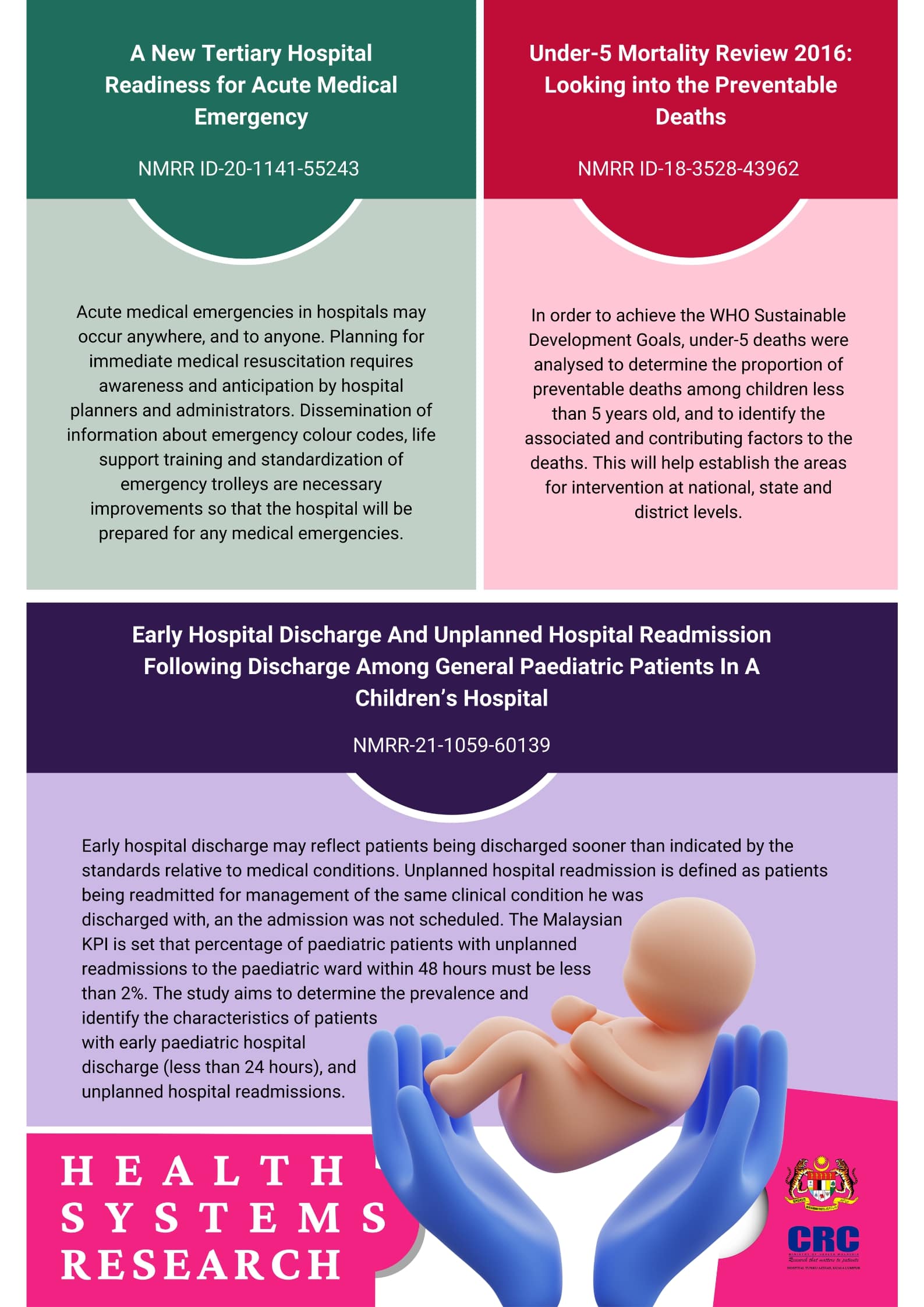
Project Highlights
Tuberculosis
The WHO’s End TB Strategy is a part of United Nation’s Sustainable Development Goals. In order to achieve the aims, there is a need for strengthening of diagnosis and effective management of tuberculosis in children. Clinical research and audit is necessary to guide clinicians to accomplish these goals.

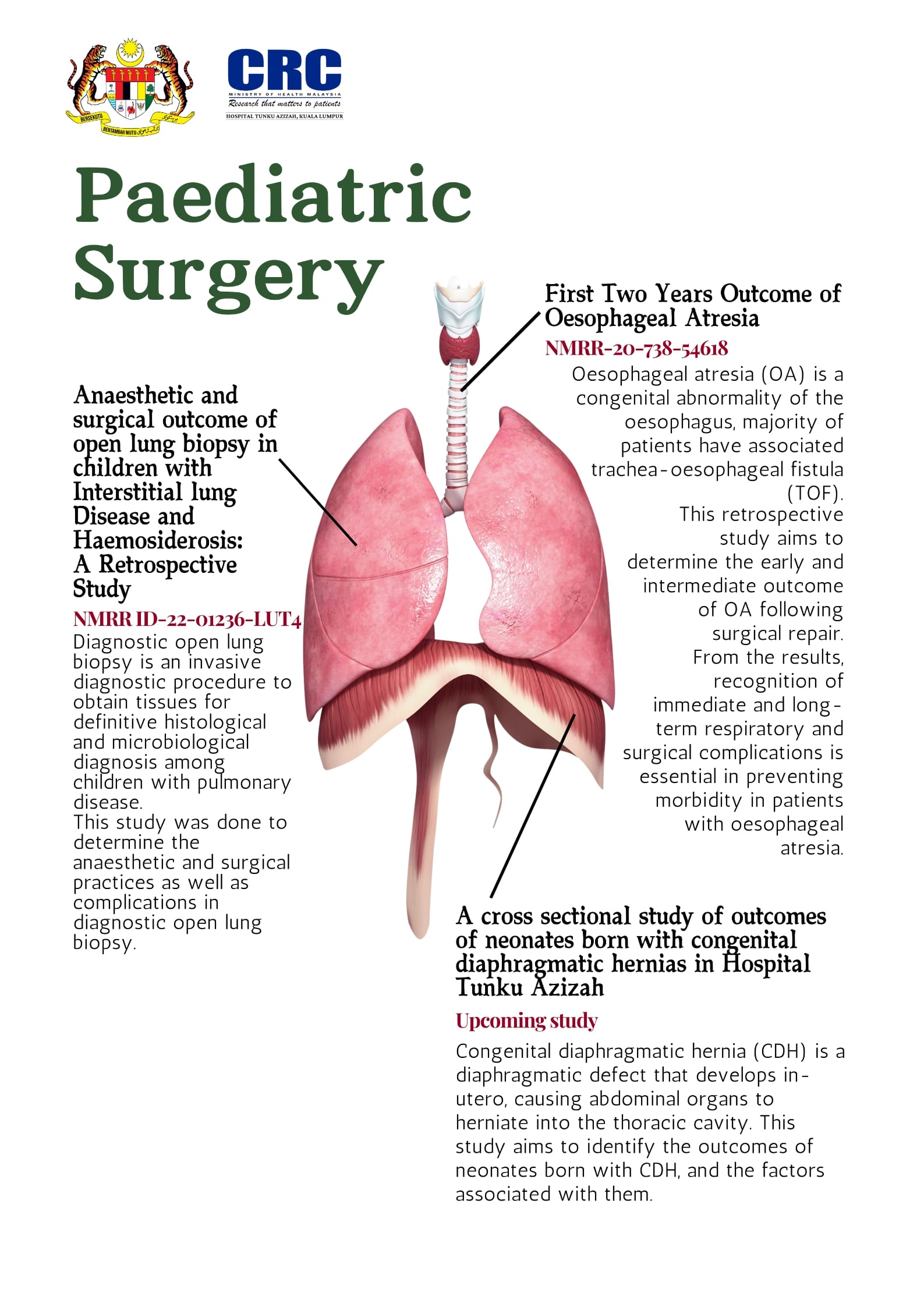
Paediatric surgery
Being an important referral centre in paediatric surgery, the hospital encounters numerous cases that are rarely seen in other centres. The studies done in collaboration with the Paediatric Surgery department not only highlights the range of cases but also the skills of the surgeons in this hospital.
Innovation and technology research
Innovation and technology in healthcare has the potential to improve diagnosis and patient management, as well as assist the hospital support system to become more efficient. As the government aims to encourage healthcare innovation, CRC Hospital Tunku Azizah supports this aspiration by providing a space for testing and research.

Rare lung diseases
As referral centres for respiratory diseases and genetic pathology, CRC Hospital Tunku Azizah worked hand in hand with the genetic laboratory and Institut Perubatan Respiratory to study rare lung diseases like bronchiectasis, childhood interstitial lung diseases, bronchiolitis obliterans, adult interstitial lung diseases, cystic fibrosis and Primary Ciliary Dyskinesia.

Diagnostic procedures
Flexible bronchoscopy is an important diagnostic and therapeutic tool in paediatric respiratory medicine, while impulse oscillometry is a non-invasive technique for measuring lung function during tidal breathing. Both established and new methods of diagnosis require research into their effectiveness and safety.

Common respiratory diseases
Asthma negatively affects children’s life through school days lost, lifestyle restrictions, frequent clinic follow-ups and emergency care. Asthma control studies are important to ensure morbidity reduction with efficient use of resources. Chronic obstructive pulmonary disease (COPD) is studied in young adults as another problem that could occur in this population.

Respiratory infections in children
Respiratory infection is one of the leading cause of mortality and the most common reason for hospital admission in paediatric population. Understanding the aetiology and burden of the disease will guide clinicians on prevention and management of this important aspect in paediatric medicine.

| NMRR-19-3887-52245 | Nasopharyngeal Carriage of Streptococcus Pneumoniae in Healthy population in Malaysia |
| NMRR-19-3889-51527 | Invasive pneumococcal disease among children 5 years old and below admitted into Malaysian public hospitals |
| NMRR-20-1378-55292 | Respiratory Outcome of Premature Newborns During the First Five Years of Life |
| NMRR-20-1187-53192 | Whole exome sequencing of childhood interstitial lung disease in Malaysia |
| NMRr-21-237-58527 | Reliability of FIBOD Balance System in assessing stability indices in healthy adults |
| NMRR-21-469-58687 | Recurrent pneumonia in children: Clinical profile and risk factors |
| NMRR-22-00074-BVO | Effect of COVID-19 pandemic to the hospital admission profile: Retrospective record review in a tertiary hospital |
| NMRR-22-02465-GBJ | Cystic fibrosis and Primary Ciliary Dyskinesia in Malaysia: Clinical Characteristic and Genetic Profile |
| NMRR ID-22-02481-2II | Indications, findings, and complications of Flexible Bronchoscopy on children in a tertiary hospital |
| NMRR-23-02109-0GW | Bronchiectasis among children in Malaysia: A multicentre, observational study |
| NMRR-23-03180-O4X | Causes of Recurrent Pneumonia |
| NMRR-23-00834-LVR | Prevalence of antenatal tobacco smoke exposure among pregnant women: A cross-sectional study |
| NMRR-23-01271-WC2 | Antenatal tobacco smoke exposure effect on newborns and post-natal smoke exposure intervention to respiratory illness in infants. |
| NMRR-24-01382-SEK | Retrospective longitudinal study on interstitial lung diseases in Malaysia (RELOS-ILD) |
| NMRR-15-1121-26592 | Phase 3, prospective, multi-center, open label study to investigate safety, immunogenicity, and hemostatic efficacy of PEGylated Factor VIII (BAX 855) in previously untreated patients (PUPs) < 6 years with severe hemophilia A (FV111 < 1%) |
| NMRR ID-21-01964-JHU | A Phase 3, Double-blind, Randomized, Placebo-Controlled , Multicenter Study Evaluating the Efficacy and Safety of Mitapivat in Subject With Non-Transfusion-Dependent Alpha -or Beta-Thalassemia (ENERGIZE) |
| NMRR ID-21-01965-4JL | A Phase 3, Double-blind, Randomized, Placebo-Controlled , Multicenter Study Evaluating the Efficacy and Safety of Mitapivat in Subject With Non-Transfusion-Dependent Alpha -or Beta-Thalassemia (ENERGIZE-T) |
| NMRR ID-21-01987-TBD | A randomized, sham-controlled, double-blind study to evaluate the efficacy and safety of intrathecal (IT) OAV101 in patients with later onset Type 2 spinal muscular atrophy (SMA) who are ≥ 2 to < 18 years of age, treatment naive, sitting, and never ambulatory |
| NMRR-21-1566-60179 | A Phase 3, Multicenter, Randomized, Partially Blinded, Palivizumab-Controlled Study to Evaluate the Safety, Efficacy and Pharmacokinetics of MK-1654 in Infants and Children at Increased Risk for Severe RSV Disease. |
| NMRR-18-3266-45336 | Phase 3, Randomized, Open-label, Controlled, Multiple-Dose, Efficacy, Safety, Pharmacokinetic,and Pharmacodynamic Study of Etelcalcetide in Pediatric Subjects 28 days to < 18 Years of age With Secondary Hyperparathyroidism and Chronic Kidney Disease Receiving Maintenance Hemodialysis |
| NMRR ID-23-00059-MKN | Evasayil: A placebo-controlled tryial to evaluate the efficacy and safety of spesolimab in the treatment of patients with Netherton syndrome |
| NMRR ID-23-03026-I7U | Long –term Follow up of Patients with Spinal Muscular Atrophy Treated with OAV101 IT or OAV101 IV in Clinical Trials |
| NMRR-24-00106-V9C | Open-label study investigating efficacy, safety and pharmacokinetics of concizumab prophylaxis in children below 12 years with haemophilia A or B with or without inhibitors. |
| NMRR-20-960-54712 | An extension study to evaluate the long-term outcomes of subject who received treatment of retinopathy of prematury in study 20090 (Study 20275). |
List of Publications associated with CRC HTA
Services at CRC Hospital Tunku Azizah
CRC HTA provides the following services:
- Research
- Initiation of and direct involvement in research activities
- Coordination, and be direct involvement with, research and innovation projects under Projek Inovasi dan Penyelidikan Kementerian Kesihatan Malaysia
- Coordination, and be direct involvement with, multicentre research projects in Malaysia
- Facilitating Clinical Research Malaysia (CRM) with Industry Sponsored Research (ISR) activities
- Consultation
- Provision of research consultation to ensure a good research proposal write-up with scientifically valid and ethically sound methodology
- Aiding researchers on sample size calculation in order to get a significant result for their projects
- Guiding researchers about registration with National Medical Research Registry (NMRR) as well as with the hospital and departments.
- Giving statistical consultation to researchers in order to optimize the output of their research
- Training
- Provision of training for hospital and CRC staff, for example, workshops and seminars, in areas related to clinical research
- Collaborating with other organizations on research-related training
- Taking part in hospital Continuous Medical Education (CME) programs
- Management
-
- Daily administrative activities related to research and to the hospital
- Implementation of research and hospital standard operating procedures
- Availability of meeting rooms for meetings related to clinical research
Meet Our Collaborators
Interdepartmental Collaborations:
- Paediatric Department
- Paediatric Surgery Department
- Obstetrics and Gynaecology Department
- Nursing Unit
- Psychiatry Department
- Radiology Department
- Hospital Administrators
- Pathology Department
- Anaesthesiology Department
- Physiotherapy Unit
Institutional Collaborations:
Institute for Medical Research (IMR)
Institut Perubatan Respiratori
Paediatric Departments from Multicentre Ministry of Health hospitals
Internal Medicine Departments from Multicentre Ministry of Health hospitals
Sektor Kawalan Tibi dan Kusta, Ministry of Health
Ministry of Health Community Clinics
Sports Medicine Unit, Hospital Kuala Lumpur
Radiology Department, Hospital Kuala Lumpur
National Cancer Institute, Ministry of Health
Hospital University Collaborations:
- University of Malaya
- Universiti Kebangsaan Malaysia
- Universiti Putra Malaysia
International Collaborations:
- The Global Health Research Unit on Respiratory Health (RESPIRE) Collaborators
- The Queen’s Medical Research Institute, University of Edinburgh
- PC-COS Children Study Group
Intitutional Collaborations




Upcoming Activities



CRC Hospital Tunku Azizah, Kuala Lumpur
Email: researchwchkl@moh.gov.my